FACTS 6.2.0 Release Notes
June 18, 2018
1 Introducing FACTS 6.2.0
Berry Consultants is delighted to announce that FACTS 6.2.0 is ready for release!
Building on FACTS 6.1.0, FACTS 6.2.0 adds new features to “FACTS N-CRM”, the ability to generate a “Design Report” from FACTS Core designs and extending the ability to compute predictive probabilities to FACTS Core TTE and FACTS Staged TTE.
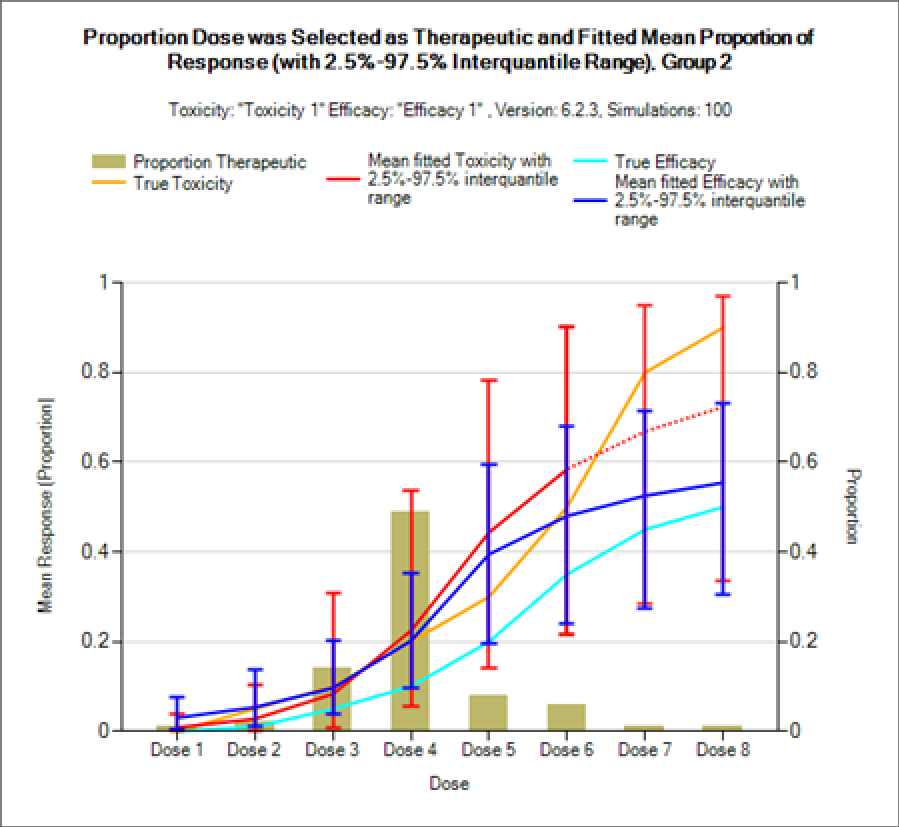
- FACTS N-CRM extensions. FACTS has had versions of the CRM with an efficacy endpoint, ordinal toxicity endpoint and 2 groups since its inception. But these were in separate engines and used the old CRM model for analysis. We have now added all these features as options to the N-CRM so they can be used with the 2 parameter Bayesian Logistic Regression model, targeting toxicity bands and the option to use overdose control. These features cannot only now but used with this better methodology, but can be used in combination with each other, and in combination with the other advanced features that were already included in the FACTS DE N-CRM simulator such as, run ins, stopping rules, escalation rules, fine grain dosing and open enrollment.
- FACTS Design Report. In FACTS Core there is now the ability to generate a “Design Report” as a MS Word file that describes the design and simulation results. The file is not intended as the final article but as something where the bulk of the straightforward text (and equations) have been provided and should just require polishing, particularly with the details of the indication and trial setting that FACTS is inevitably unaware of.

- FACTS 6.2 completes the implementation of predictive probabilities. Predictive probabilities in the current trial with a TTE endpoint are considerably more complex than predictive probabilities in the other endpoints. For the other endpoints the expected about of information after full enrollment and full follow-up is known, for time-to-event it can depend on multiple things such as accrual rate and the expected number of events so a degree of simulation within the simulation is required.
FACTS 6.2.0 is fully backwards compatible with FACTS 6.1.0, 6.0.0 and 5 – it can load and run all your FACTS 6.0.0 and FACTS 5 designs – and then use new FACTS 6.2.0 features with those designs. You can have FACTS 6.2.0 and FACTS 6.1.0 installed on the same machine, so it’s easy to have a transition period as you move to the new version.
2 Key New Features
- FACTS Dose Escalation, the N-CRM (also known as Bayesian Logistic Regression) now has options for:
- An ordinal toxicity endpoint
- To simulate a trial across 2 groups (e.g. Adults and Pediatrics)
- An additional binary Efficacy endpoint
- These options can be combined with each other and all the other N-CRM options.
- FACTS Core TTE
- The ability to compute the predictive probability of success at the full enrollment of the current trial.
- FACTS Staged Design TTE
- The ability to compute the predictive probability of success
- in Stage 1 at full enrollment
- of Stage 2 (whilst in Stage 1)
- in Stage 2 at full enrollment (whilst in Stage 2).
- The ability to compute the predictive probability of success